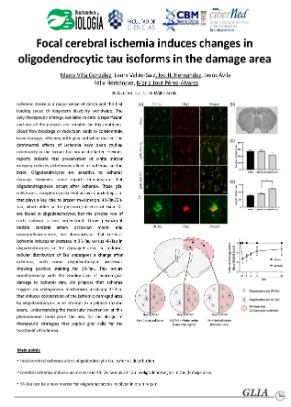
Ischemic stroke is a major cause of death and the first leading cause of long‐term disability worldwide. The only therapeutic strategy available to date is reperfusion and not all the patients are suitable for this treatment. Blood flow blockage or reduction leads to considerable brain damage, affecting both gray and white matter. The detrimental effects of ischemia have been studied extensively in the former but not in the latter. Previous reports indicate that preservation of white matter integrity reduces deleterious effect of ischemia on the brain. Oligodendrocytes are sensitive to ischemic damage, however, some reports demonstrate that oligodendrogenesis occurs after ischemia. These glial cells have a complex cytoskeletal network, including tau, that plays a key role to proper myelination. 4R‐Tau/3R‐Tau, which differ in the presence/absence of Exon 10, are found in oligodendrocytes; but the precise role of each isoform is not understood. Using permanent middle cerebral artery occlusion model and immunofluorescence, we demonstrate that cerebral ischemia induces an increase in 3R‐Tau versus 4R‐Tau in oligodendrocytes in the damaged area. In addition, cellular distribution of Tau undergoes a change after ischemia, with some oligodendrocytic processes showing positive staining for 3R‐Tau. This occurs simultaneously with the amelioration of neurological damage in ischemic rats. We propose that ischemia triggers an endogenous mechanism involving 3R‐Tau, that induces colonization of the ischemic damaged area by oligodendrocytes in an attempt to myelinate‐injured axons. Understanding the molecular mechanism of this phenomenon could pave the way for the design of therapeutic strategies that exploit glial cells for the treatment of ischemia.
Universidad Autónoma de Madrid © 2008 · Ciudad Universitaria de Cantoblanco · 28049 Madrid · Información y Conserjería: 91 497 43 31 E-mail: informacion.ciencias@uam.es Gestión de estudiantes de Grado y Posgrado: 91 497 8264 / 4329 / 4353 / 4349 / 6879 / 8362 E-mail: administracion.ciencias@uam.es